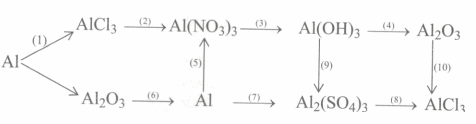
Đề bài
Viết các phương trình hoá học để hoàn thành chuỗi biến hoá sau :
Lời giải chi tiết
\(\eqalign{
& (1)\,2Al\, + 3C{l_2}\xrightarrow{{{t^o}}}\,\,2AlC{l_3} \cr
& (2)\,\,AlC{l_3}\,\,\, + \,\,\,3AgN{O_3}\, \to \,\,3AgCl\,\, + \,\,Al{(N{O_3})_3} \cr
& (3)\,\,Al{(N{O_3})_3}\,\, + \,\,\,3KOH\,\, \to \,Al{(OH)_3}\,\, \downarrow \,\, + \,\,3KN{O_3} \cr
& (4)\,\,Al{(OH)_3}\,\xrightarrow{{{t^o}}}\,\,\,A{l_2}{O_3}\, + \,3{H_2}O \cr
& (5)\,2Al\,\, + \,\,3Cu{(N{O_3})_2} \to 2Al{(N{O_3})_3}\, + \,\,3Cu \cr
& (6)\,\,2A{l_2}{O_3}\,\xrightarrow{{{xt, t^o}}}\,\,4Al\,\, + \,\,3{O_2} \cr
& (7)\,\,2Al\,\,\, + \,\,3{H_2}S{O_4}\,\text{(loãng)}\,\, \to \,\,A{l_2}{(S{O_4})_3}\,\, + \,\,3{H_2} \uparrow \cr
& (8)\,\,A{l_2}{(S{O_4})_3}\,\, + \,\,3BaC{l_2}\,\, \to \,BaS{O_4} \downarrow \,\, + \,\,2AlC{l_3} \cr
& (9)\,\,Al{(OH)_3}\, + \,3{H_2}S{O_4}\, \to \,\,A{l_2}{(S{O_4})_3}\,\, + \,\,3{H_2}O \cr
& (10)\,A{l_2}{O_3}\, + \,6HCl\,\, \to \,\,2AlC{l_3}\,\, + \,\,3{H_2}O \cr
& (11)\,4Al\,\, + \,\,\,3{O_2}\,\,\xrightarrow{{{t^o}}}\,\,2A{l_2}{O_3}\, \cr} \)
soanvan.me